
Our network
Over the years BRM Extremities has created a sales network, made up of distributors selected internationally, with the aim of responding to the logistical needs of final costumers.
The expertise in Regulatory and certification of devices, in the EC (according to Directive 93/42 EEC), FDA and ANVISA, allow the company to satisfy every type of request in the field of trauma and joint surgery of short bones, guaranteeing the highest standards of device quality and efficacy and patient safety.
The basic requirements necessary to become part of the company's distribution network are of a technical, regulatory and ethical nature. The experience and the company structure, the turnover and the ethical code are all fundamental criteria for the selection of our partners.
BRM Extremities guarantees, on the other hand, territorial exclusivity and logistic support as well as a complete range of products for the extremities and competitive delivery times. One of the main strengths of the partnership is the specific training on the product addressed to both the distributor and the end user.

Trainings

Workshops

Monothematic Congresses

Cadaver Lab

National Congresses

Events
Discover
Our partner program
Being part of the network of distributors means having access to a high quality and highly competitive made in Italy product. Our distributors operate in multiple parts of the world, including the United States, South America and Europe.
Fill out the form and download the information material containing all the necessary requirements to become part of our distribution network.
The entrepreneurial experience, the design skills and the knowledge of production technologies, as well as of the clinical applications of the sector, are the strengths of the company.
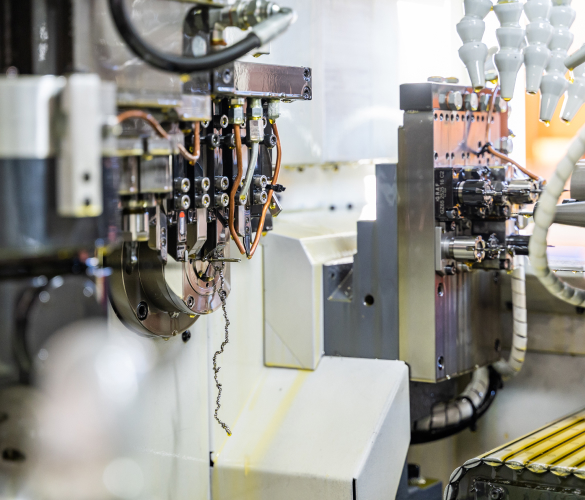
The production unit
The plant located in the province of Lecco is the beating heart of BRM production. The most recent CNC and CAD / CAM technologies allow the creation of cutting-edge products and the presence of a cleanroom, dedicated to washing and packaging the devices, guarantees an aseptic environment. BRM Extremities is ISO 13485: 2016 and ISO 9001: 2015 certified.
Certificates
The expertise in Regulatory and device certification, in the CE, FDA and ANVISA fields, allow the company to satisfy every type of request in the field of trauma and joint surgery of short bones, guaranteeing the highest standards of quality and effectiveness of the devices as well as of the patient safety Discover all the Quality Management certificates.

